News & Event
Biopson Receives MFDS Medical Device Manufacturer & Manufacturing Approval
DATE Jun 22, 2023
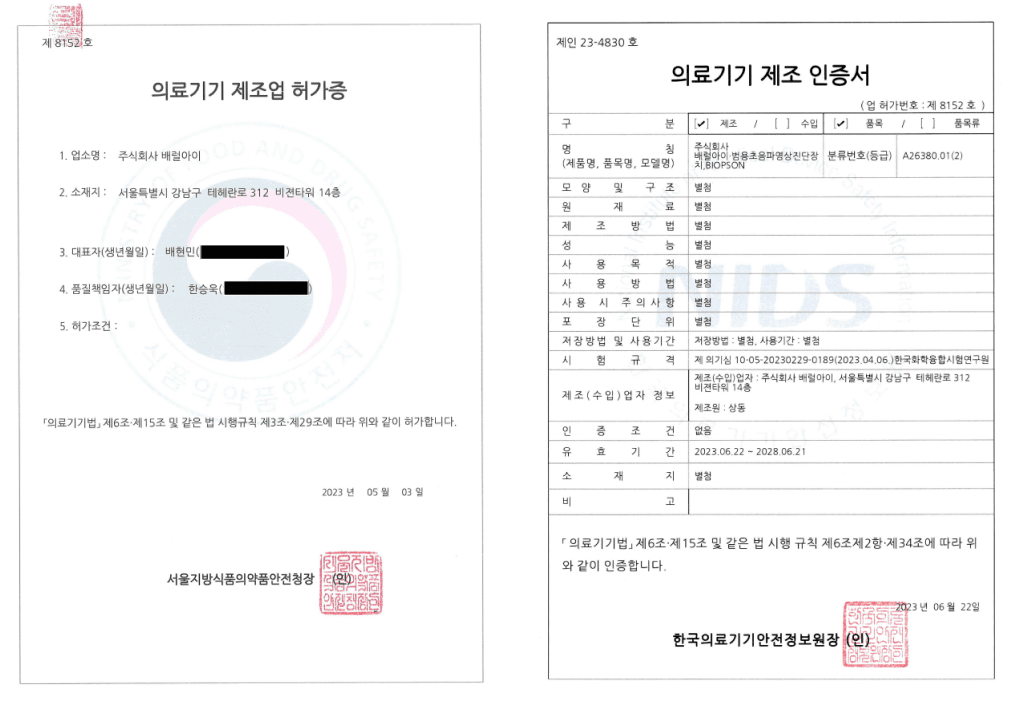
Barreleye Inc. has obtained both the Medical Device Manufacturer License and the Medical Device Manufacturing Certificate from the Ministry of Food and Drug Safety (MFDS) and the Korea Medical Device Safety Information Center, authorizing the company to manufacture and manage the quality of the Biopson quantitative ultrasound diagnostic device.
Certification Details
- Medical Device Manufacturer License
• License No.: 8152
• Date of Issue: May 3, 2023
• Issued by: Commissioner, Ministry of Food and Drug Safety (MFDS)
- Medical Device Manufacturing Certificate
• License No.: IN23-4830
• Date of Issue: June 22, 2023
• Issued by: President, Korea Medical Device Safety Information Center
- News
